Team 2: Cellular and Molecular Cardiac Physiopathology
Principal Investigator
Aniella Abi-Gerges, PhD
Associate Professor of Physiology
Gilbert and Rose-Marie Chagoury School of Medicine
Lebanese American University - Byblos Campus
Office: 4706
Telephone: +961 9 547254; Ext. 2843
Email: aniella.abigerges@lau.edu.lb
Research Topic Summary
Cardiovascular diseases (CVDs) are the leading cause of mortality in the world with an estimated 17.5 million deaths reported in 2012 - a number which is expected to grow to more than 23.6 million by 2030. Heart failure (HF), the last stage of most CVDs and a highly morbid and mortal condition, is characterized by a functional impairment of heart function. Despite continuous advances in treatment and prevention strategies, specific pharmacologic therapy reversing HF is not yet available.
Diabetes mellitus (DM) contributes to the development of HF independently of the conventional CVD risk factors such as coronary artery disease and hypertension. The clinical outcomes associated with HF are considerably worse for patients with diabetes than for non-diabetic patients. Indeed, hyperglycemia, hyperlipidemia and disrupted insulin signaling can concur to the development of diabetic cardiomyopathy (DCM). The latter comprises a spectrum of cardiac abnormalities including cardiac hypertrophy (CH), myocardial fibrosis, diabetic microangiopathy, diastolic and systolic cardiac dysfunction. The prevalence of DCM is increasing in parallel with the increase in diabetes and CVDs remain the most common cause of mortality among patients with diabetes. Despite continuous advances in treatment and prevention strategies, specific pharmacologic therapy reversing HF is not yet available. Moreover, HF associated with diabetes has received little attention and the pathophysiology of DCM is still not fully understood.
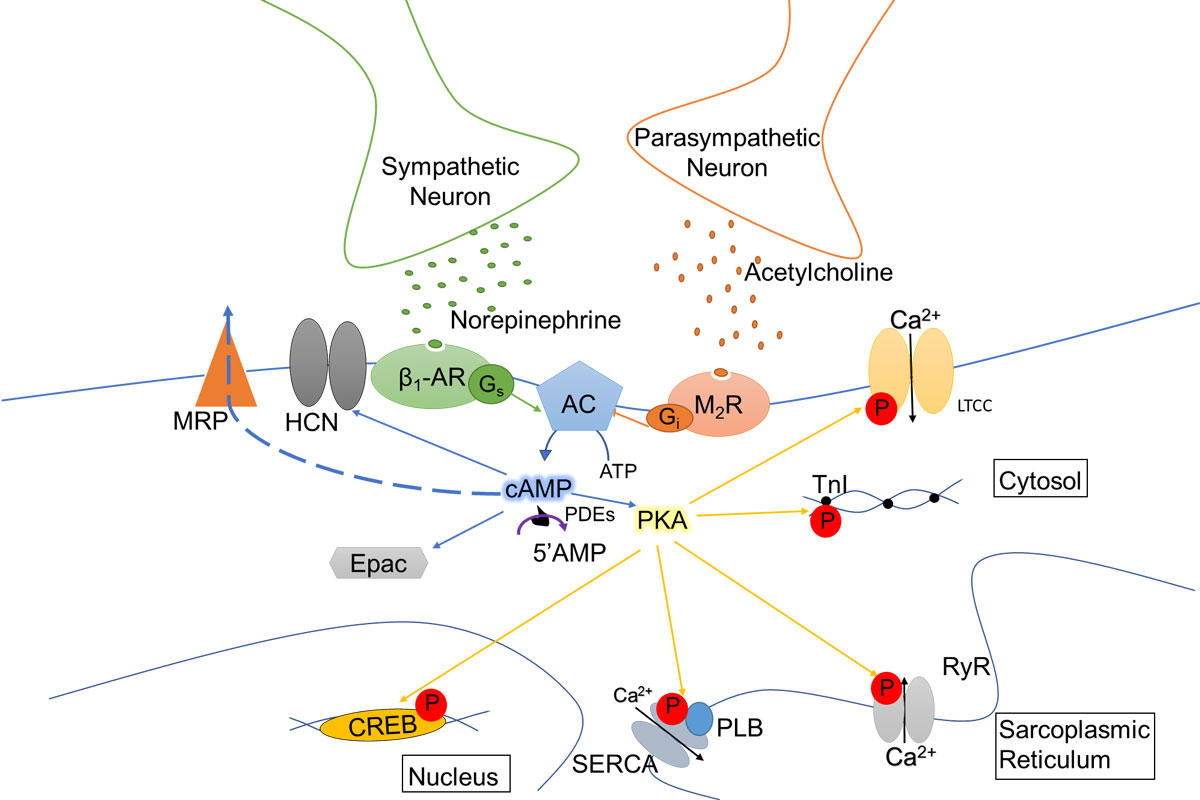
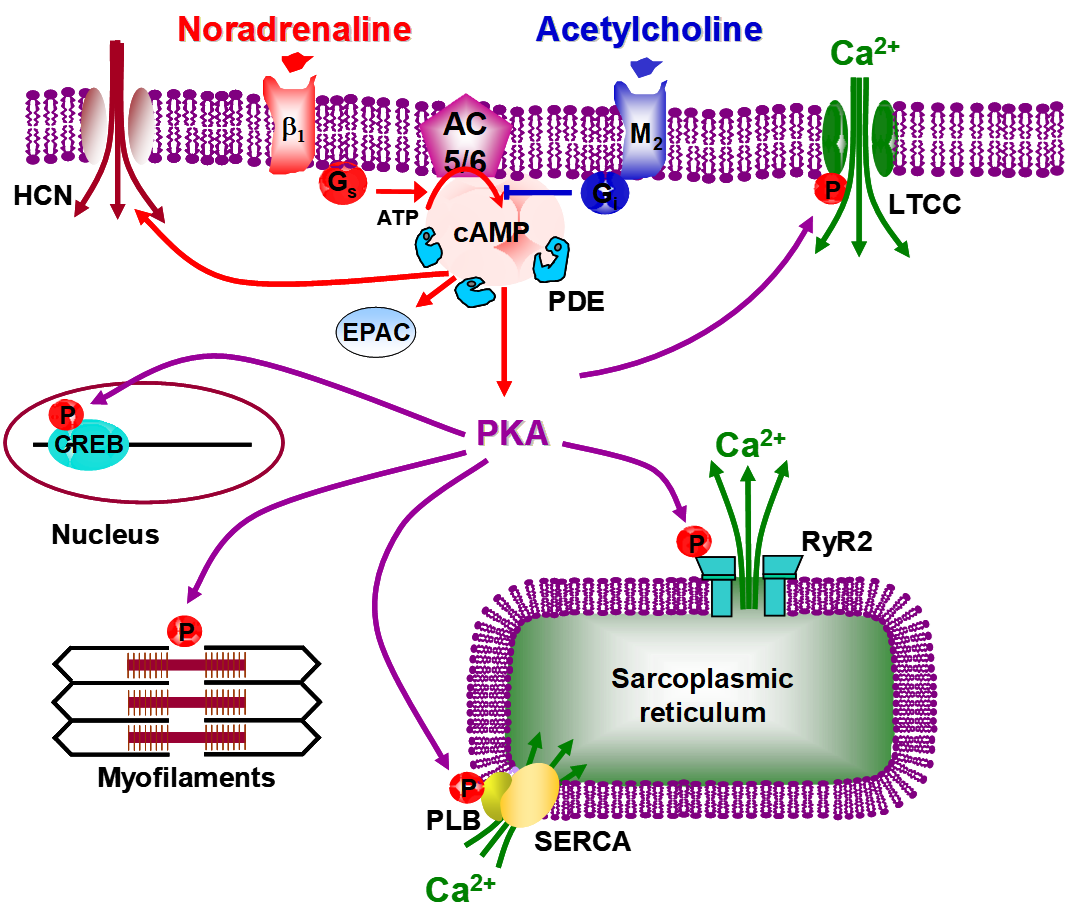
cAMP pathway in normal cardiac myocytes. Upon liberation of noradrenaline by sympathetic nerve terminals, ß1-adrenergic receptors
(ß1-ARs) activate cardiac adenylate cyclase (AC) isoforms (AC5 and AC6) via the stimulatory G protein (Gs). Cyclic AMP is synthesized and activates cAMP-dependent protein kinase (PKA), which phosphorylates different intracellular targets: L-type Ca2+ channels (LTCC) phosphorylation leads to an increased Ca2+ influx and ryanodine receptors (RyR2) phosphorylation at the sarcoplasmic reticulum (SR) membrane leads to an increased Ca2+induced Ca2+ release, both acting to enhance the force of contraction; phospholamban (PLB) phosphorylation leads to an increased SR Ca2+ uptake by the the sarcoplasmic reticulum (SR) Calcium (Ca2+) pump (SERCA2a) and troponin I (TnI) phosphorylation leads to a reduction in Ca2+ sensitivity of the myofilaments, both acting to accelerate contractile relaxation. On the long term, CREB phosphorylation in the nucleus activates transcription. These effects are antagonized by a liberation of acetylcholine from parasympathetic nerve terminals, through activation of muscarinic M2 receptors and inhibition of AC activity via inhibitory G proteins (Gi). cAMP is able to activate directly Epac, a guanine nucleotide-exchange factor for the small GTPase Rap1, and HCN cyclic nucleotide-gated ion channels. cAMP is degraded by cyclic nucleotide phosphodiesterases (PDEs) into 5’AMP. By degrading cAMP, PDEs not only terminate hormonal signals but also limit the spread of this messenger thereby shaping and organizing intracellular-signaling microdomains that maintain specificity of cellular response. Thus, altered PDE expression/activity and consequently disrupted cAMP signaling play key roles in the pathogenesis of many clinically relevant disease states, including obesity, diabetes, cardiac hypertrophy and heart failure.
Pathway in normal cardiac myocytes
In normal myocardium, cyclic adenosine 3’-5’ monophosphate (cAMP) modulates a myriad of processes, from short-term action in contraction/relaxation to long-term effects on growth/survival. cAMP elevation, particularly through β-adrenergic receptor (β-AR) stimulation, exerts inotropic and lusitropic effects by activating cAMP-dependent protein kinase A (PKA). This latter phosphorylates key proteins of cardiac excitation-contraction coupling (ECC) and modulates their function. cAMP also binds and actives the exchange protein Epac which mediates pro-hypertrophic effects of β-AR stimulation.
Intracellular cAMP content is a balance between its synthesis by adenylate cyclase (AC) and degradation by cardiac cyclic nucleotide phosphodiesterases (PDEs) which fall into seven families (PDE1-PDE5, PDE8, PDE9) and are found in specific intracellular locations. By degrading cAMP, PDEs terminate hormonal signals and prevent global rises in cAMP, known to be deleterious, thereby shaping intracellular-signaling microdomains that maintain cellular responses specificity. Thus, altered PDE expression/activity and consequently disrupted cAMP signaling play key roles in the pathogenesis of many clinically relevant disease states, including obesity, diabetes, CH and HF. Additionally, cAMP is extruded from adult cardiac myocytes by an ATP-dependent transport mechanism involving multidrug-resistance protein 4 (MRP4)
Impairment of cAMP-mediated signaling is a molecular hallmark of cardiac hypertrophy and heart failure, attributed to chronic activation of the sympathetic nervous system. However, alterations affecting cAMP pathway and Ca2+ signaling/ECC in DCM are controversial.
The main focus of the team’s research is to examine specific changes affecting the cardiac β-AR/cAMP and calcium pathways in DCM and evaluate their consequences on cardiac function and the major actors involved in ECC. Understanding the molecular mechanisms underlying DCM development by tackling these pathways would lead to a better management of diabetic patients and prevent the progression of DCM to HF.
Team Members
Pamela Abou Khalil
Senior Science Lab Technician
Gilbert and Rose-Marie Chagoury School of Medicine
Lebanese American University, Byblos Campus
Office: 4718
Telephone: +961 9 547254, ext. 2993
Email: pamela.aboukhalil@lau.edu.lb
Marianne Bejjani, Research Assistant
Email: marianne.bejjani@lau.edu.lb
Research Collaborators
Grégoire Vandecasteele, PhD, FISHR
INSERM UMR-S1180 Signaling and Cardiovascular Pathophysiology
Laboratory of Excellence in Drug Discovery and Therapeutic Innovation (Labex LERMIT)
Université Paris-Saclay, Orsay, France
Christian Khalil, PhD
School of Arts and Sciences, Lebanese American University
Byblos, Lebanon
Selected Publications
Victoria Chaoul, Rita Hanna, Pia Hachem, Magali Samia El Hayek, Wared Nour-Eldine, Pamela Abou-Khalil, Elias Abi-Ramia, Grégoire Vandecasteele, Aniella Abi-Gerges. Differential changes in cyclic adenosine 3′-5′ monophosphate (cAMP) effectors and major Ca2+ handling proteins during
diabetic cardiomyopathy (DCM). Journal of Cellular and Molecular Medicine (2023) 27(9):1277-1289.
Wared Nour-Eldine, Katia Sayyed, Zeina Harhous, Carole Dagher-Hamalian, Stephanie Mehanna, Donna Achkouti, Hanan ElKazzaz, Rony S. Khnayzer, Firas Kobeissy, Christian Khalil, Aniella Abi-Gerges. Gasoline fume inhalation induces apoptosis, inflammation, and favors Th2 polarization in C57BL/6 mice. Journal of Applied Toxicology (2022) 42(7):1178-1191.
Rita Hanna, Wared Nour-Eldine, Youakim Saliba, Carole Dagher-Hamalian, Pia Hachem^, Pamela Abou-Khalil, Delphine Mika, Audrey Varin, Magali Samia El Hayek^, Laetitia Pereira, Nassim Fares, Gregoire Vandecasteele, Aniella Abi-Gerges. Cardiac phosphodiesterases are differentially increased in diabetic cardiomyopathy. Life Sciences (2021) 283:119857.
Aniella Abi-Gerges, Liliana Castro, Jérôme Leroy, Valérie Domergue-Dupont, Rodolphe Fischmeister, Grégoire Vandecasteele. Selective changes in cytosolic β-adrenergic cAMP signals and L-type Calcium Channel regulation by Phosphodiesterases during cardiac hypertrophy. Journal of Molecular and Cellular Cardiology (2021) 150:109-121.
Aniella Abi-Gerges, Carole Dagher-Hamalian, Pamela Abou-Khalil, Joe Braham Chahine^, Pia Hachem^, Christian Khalil. Evaluation of waterpipe smoke toxicity in C57BL/6 mice model. Pulmonary Pharmacology and Therapeutics (2020) 63:101940.
Yassine Sassi, Aniella Abi-Gerges, Jeremy Fauconnier, Nathalie Mougenot, Steven Reiken, Kobra Haghighi, Evangelia G Kranias, Andrew R. Marks, Alain Lacampagne, Stefan Engelhardt, Stéphane N Hatem, Anne-Marie Lompré, Jean-Sébastien Hulot. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. Faseb Journal (2012) 26(3):1009-17.
Jérôme Leroy, Wito Richter, Delphine Mika, Liliana RV Castro, Aniella Abi-Gerges, Moses Xie, Colleen Scheitrum, Florence Lefebvre, Julia Schittl, Ruth Westenbroek, William A. Catterall, Flavien Charpentier, Marco Conti, Rodolphe Fischmeister, and Grégoire Vandecasteele. Phosphodiesterase 4B in the cardiac L-type Ca2+ channel complex regulates Ca2+ current and protects against ventricular arrhythmias in mice. Journal of Clinical Invesigation, (2011) 121(7):2651-2661.
Aniella Abi-Gerges, Wito Richter, Florence Lefebvre, Christophe Heymes, Jane-Lise Samuel, Claire Lugnier, Marco Conti, Rodolphe Fischmeister, and Grégoire Vandecasteele. Decreased expression and activity of cAMP phosphodiesterases in cardiac hypertrophy and its impact on β-adrenergic cAMP signals. Circulation Research, (2009) 105(8):784-92.
Jérôme Leroy, Aniella Abi-Gerges, Viacheslav O Nikolaev, Wito Richter, Jean-Luc Mazet, Marco Conti, Rodolphe Fischmeister, and Grégoire Vandecasteele. Spatiotemporal dynamics of β-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: Role of phosphodiesterases. Circulation Research (2008) 102: 1091-100.
Rochais, F., Abi-Gerges, A., Horner, K., Lefebvre, F., Cooper, M. F., Conti, M., Fischmeister, R. & Vandecasteele, G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circulation Research (2006) 98, 1081-1088.
Grants
- National Council for Scientific Research (CNRS) and Lebanese American University, 2018-2020: Characterization of cAMP signaling pathway in diabetic cardiomyopathy: Interplay between PDEs, MRP4 and Epac.
- Partenariat Hubert Curien franco-libanais - PHC CEDRE, 2019-2020: Rôle des régulateurs de la voie β-adrénergique dans la cardiomyopathie diabétique et les arythmies.
- LAU President’s Intramural Research Fund, 2022-2024: Effects of waterpipe smokes on cardiac cyclic adenosine 3’-5’ monophosphate (cAMP) signaling and the major actors of the cardiac excitation contraction coupling (ECC)
in diabetic cardiomyopathy (DCM).
Public Exposure
جهد أنيلا أبي جرجس بأخلاق الطب وحرّية المريض (almodon.com)
The Lebanese Physician’s podcast:
https://www.youtube.com/watch?si=BgH-PNlRkRuDeYRB&v=3Pyp0bEkrBo&feature=youtu.be